Snapshot Science: Is a New FDA-Approved Transcatheter Pulmonary Valve Safe and Effective for Patients?
The findings:
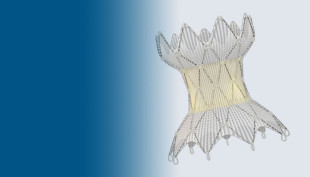 The Harmony Transcatheter Pulmonary Valve™ (TPV) is the first device approved by the U.S. Food and Drug Administration (FDA) to treat severe pulmonary regurgitation in the heart’s right ventricular outflow tract (RVOT). After one year, the device proved to be safe and effective, according to an analysis of three, multisite clinical trials led by a principal investigator at Children’s Hospital of Philadelphia. The study showed that 98% of patients who received the TPV22 model of the device and 91% of patients who received the TPV25 model were free from pulmonary regurgitation, stenosis (a narrowing of the main pulmonary artery), and reintervention. (22 and 25 refer to the size of the diameter of the device.)
The Harmony Transcatheter Pulmonary Valve™ (TPV) is the first device approved by the U.S. Food and Drug Administration (FDA) to treat severe pulmonary regurgitation in the heart’s right ventricular outflow tract (RVOT). After one year, the device proved to be safe and effective, according to an analysis of three, multisite clinical trials led by a principal investigator at Children’s Hospital of Philadelphia. The study showed that 98% of patients who received the TPV22 model of the device and 91% of patients who received the TPV25 model were free from pulmonary regurgitation, stenosis (a narrowing of the main pulmonary artery), and reintervention. (22 and 25 refer to the size of the diameter of the device.)
Why it matters:
Congenital heart disease is the most common birth defect encountered across the world. Tetralogy of Fallot is a combination of four congenital heart defects: a hole in the wall between the two lower chambers, a narrowing of the pulmonary valve and main pulmonary artery, an overriding aorta, and a thick muscular wall of the right ventricle.
Doctors can surgically repair this defect by widening the pulmonary valve and enlarging the pathway to the pulmonary artery. However, that surgery creates a secondary problem: After the valve is widened, a significant portion of blood may leak back into the right side of the heart. If left untreated, that leak — called chronic pulmonary regurgitation — can lead to heart failure. Nearly all patients who receive surgery to widen the pulmonary valve will eventually need to receive a bioprosthetic heart valve via open-heart surgery. The risks and recovery time for heart valve surgery are high, and it’s likely that the valve will need to be replaced during a patient’s lifetime.
The Harmony TPV, designed by Medtronic, is the first technology designed to non-surgically replace the pulmonary valve and treat pulmonary regurgitation. To place the hour-glass shaped device in the heart, an interventional cardiologist inserts the Harmony TPV via a catheter through a blood vessel in the leg or the neck. The device is then released from the catheter, it expands, and forces blood flow out of the heart to the lungs. The procedure is significantly less invasive than open-heart surgery, and recovery time is minimal.
Who conducted the study:
 Matthew J. Gillespie, MD, an attending cardiologist in CHOP’s Cardiac Center, is the study’s senior author. Dr. Gillespie is also director of the Cardiac Catheterization Lab and co-director of the Topolewski Heart Valve Center.
Matthew J. Gillespie, MD, an attending cardiologist in CHOP’s Cardiac Center, is the study’s senior author. Dr. Gillespie is also director of the Cardiac Catheterization Lab and co-director of the Topolewski Heart Valve Center.
How they did it:
The Harmony TPV is the first device to be tested in a clinical trial under the FDA’s early feasibility study pathway. An early feasibility study is a limited clinical investigation of a device early in development that enrolls a small number of participants, in order to evaluate and modify its initial design. Researchers initially evaluated the Harmony TPV device’s performance in a feasibility study of 20 patients from 2012 to 2015. The initial study was followed by two larger, multisite, non-randomized trials.
To assess the safety and efficacy of the device, researchers pooled data from the three patient cohorts and analyzed outcomes at the one-year mark. The analysis includes data from 87 total patients who were enrolled in the three studies. The median patient age at treatment was 26 years old for the TPV22 and 29 years old for the TPV25.
Quick thoughts:
“I think every baby born with Tetralogy of Fallot should live to be 90 years old — we have to start thinking about these patients over a long lifespan,” Dr. Gillespie said. “These newer, catheter-based technologies are going to allow us to replace the pulmonary valve in a less invasive way, and over the course of their lifetime, reduce the number of open-heart surgeries that patients need, at a minimum. At a maximum, it could eliminate the need for repeated surgeries altogether.”
What’s next:
The research team is continuing follow-up of this patient cohort through 10 years to evaluate and improve the long-term performance of the Harmony TPV device.
Where the study was published:
This study appeared in the Journal of Cardiovascular Interventions.
Disclosures:
This study was supported by Medtronic. Dr Gillespie serves as a consultant for Medtronic; as an advisory board member and a consultant for Abbott; and as a consultant and principal investigator for W. L. Gore & Associates.